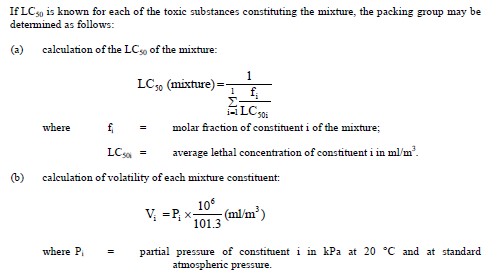
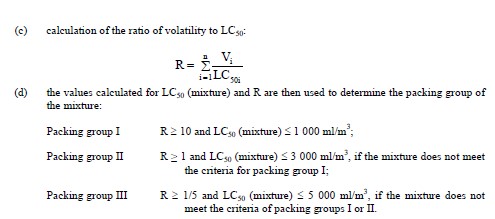
In the absence of LC50 data on the toxic constituent substances, the mixture may be assigned to a
group based on the following simplified threshold toxicity tests. When these threshold tests are used,
the most restrictive group shall be determined and used for carrying the mixture.
A mixture is assigned to packing group I only if it meets both of the following criteria:
(a) A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere
of 1 000 ml/m3 vaporized mixture in air. Ten albino rats (5 male and 5 female) are exposed to
the test atmosphere for 1 hour and observed for 14 days. If five or more of the animals die
within the 14-day observation period, the mixture is presumed to have an LC50 equal to or less
than 1 000 ml/m3;
(b) A sample of vapour in equilibrium with the liquid mixture is diluted with 9 equal volumes of
air to form a test atmosphere. Ten albino rats (5 male and 5 female) are exposed to the test
atmosphere for 1 hour and observed for 14 days. If five or more of the animals die within
the 14-day observation period, the mixture is presumed to have a volatility equal to or greater
than 10 times the mixture LC50.
A mixture is assigned to packing group II only if it meets both of the following criteria, and does not
meet the criteria for packing group I:
(a) A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere of
3 000 ml/m3 vaporized mixture in air. Ten albino rats (5 male and 5 female) are exposed to the
test atmosphere for 1 hour and observed for 14 days. If five or more of the animals die within
the 14-day observation period, the mixture is presumed to have an LC50 equal to or less
than 3 000 ml/m3;
(b) A sample of the vapour in equilibrium with the liquid mixture is used to form a test
atmosphere. Ten albino rats (5 male and 5 female) are exposed to the test atmosphere for
1 hour and observed for 14 days. If five or more of the animals die within the 14-day
observation period, the mixture is presumed to have a volatility equal to or greater than the
mixture LC50.
A mixture is assigned to packing group III only if it meets both of the following criteria, and does not
meet the criteria for packing groups I or II:
(a) A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere of
5 000 ml/m3 vaporized mixture in air. Ten albino rats (5 male and 5 female) are exposed to the
test atmosphere for 1 hour and observed for 14 days. If five or more of the animals die within
the 14-day observation period, the mixture is presumed to have an LC50 equal to or less
than 5 000 ml/m3;
(b) The vapour concentration (volatility) of the liquid mixture is measured and if the vapour
concentration is equal to or greater than 1 000 ml/m3, the mixture is presumed to have a
volatility equal to or greater than 1/5 the mixture LC50.
Methods for determining oral and dermal toxicity of mixtures
When classifying and assigning the appropriate packing group to mixtures in Class 6.1 in accordance
with the oral and dermal toxicity criteria (see 2.2.61.1.3), it is necessary to determine the acute LD50
of the mixture.
If a mixture contains only one active substance, and the LD50 of that constituent is known, in the
absence of reliable acute oral and dermal toxicity data on the actual mixture to be carried, the oral or
dermal LD50 may be obtained by the following method:
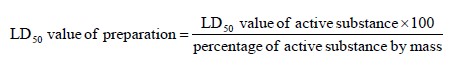
If a mixture contains more than one active constituent, there are three possible approaches that may be
used to determine the oral or dermal LD50 of the mixture. The preferred method is to obtain reliable
acute oral and dermal toxicity data on the actual mixture to be carried. If reliable, accurate data are not
available, then either of the following methods may be performed:
(a) Classify the formulation according to the most hazardous constituent of the mixture as if that
constituent were present in the same concentration as the total concentration of all active
constituents; or
(b) Apply the formula:
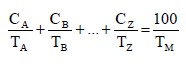
where:
C = the percentage concentration of constituent A, B, ..., Z in the mixture;
T = the oral LD50 values of constituent A, B, ... Z;
TM = the oral LD50 value of the mixture.
NOTE: This formula can also be used for dermal toxicities provided that this information is
available on the same species for all constituents. The use of this formula does not take into
account any potentiation or protective phenomena.
Classification of pesticides
All active pesticide substances and their preparations for which the LC50 and/or LD50 values are
known and which are classified in Class 6.1 shall be classified under appropriate packing groups in
accordance with the criteria given in 2.2.61.1.6 to 2.2.61.1.9. Substances and preparations which are
characterized by subsidiary risks shall be classified according to the precedence of hazard Table in
2.1.3.10 with the assignment of appropriate packing groups.
If the oral or dermal LD50 value for a pesticide preparation is not known, but the LD50 value of its
active substance(s) is known, the LD50 value for the preparation may be obtained by applying the
procedures in 2.2.61.1.10.
NOTE: LD50 toxicity data for a number of common pesticides may be obtained from the most current
edition of the document "The WHO Recommended Classification of Pesticides by Hazard and
Guidelines to Classification" available from the International Programme on Chemical Safety, World
Health Organisation (WHO), 1211 Geneva 27, Switzerland. While that document may be used as a
source of LD50 data for pesticides, its classification system shall not be used for purposes of transport
classification of, or assignment of packing groups to, pesticides, which shall be in accordance with
the requirements of ADR.
The proper shipping name used in the carriage of the pesticide shall be selected on the basis of the
active ingredient, of the physical state of the pesticide and any subsidiary risks it may exhibit (see
3.1.2).
If substances of Class 6.1, as a result of admixtures, come into categories of risk different from those
to which the substances mentioned by name in Table A of Chapter 3.2 belong, these mixtures or
solutions shall be assigned to the entries to which they belong on the basis of their actual degree of
danger.
NOTE: For the classification of solutions and mixtures (such as preparations and wastes), see
also 2.1.3.
On the basis of the criteria of 2.2.61.1.6 to 2.2.61.1.11, it may also be determined whether the nature
of a solution or mixture mentioned by name or containing a substance mentioned by name is such that
the solution or mixture is not subject to the requirements for this Class.
Substances, solutions and mixtures, with the exception of substances and preparations used as
pesticides, which are not classified as acute toxic category 1, 2 or 3 according to Regulation (EC) No
1272/20083, may be considered as substances not belonging to class 6.1.
